Hierarchical Capillarity-assisted Liquid Invasion in Multi-layered Paper Channels for Nanoelectrokinetic Preconcentration
A nanoelectrokinetic phenomenon called ion concentration polarization (ICP) has been recently applied to microfluidic paper-based devices for the high-fold preconcentration of low-concentration analytes. The inherent microstructural characteristics of cellulose papers can sufficiently stabilize the chaotic electroconvection of ICP, which is a significant annoyance for typical engineered microfluidic channels. However, a high electrical voltage to induce ICP in paper-fluidic channel can increase unavoidable electrophoretic forces over drag forces so that the preconcentrated plug is rapidly receded with severe dispersion. In order to enhance the hydraulic drag force that helps preconcentration of analytes, here we introduce a multi-layered paper structure into paper-fluidic channel. We theoretically and experimentally demonstrate that a hierarchical capillary structure in multi-layered paper-fluidic channel can effectively increase the hydraulic drag force. For the practical utility of this study, in the field of diagnostics in particular, the mechanism is verified by a simple example of the immunoassay using biotin-streptavidin complexation.
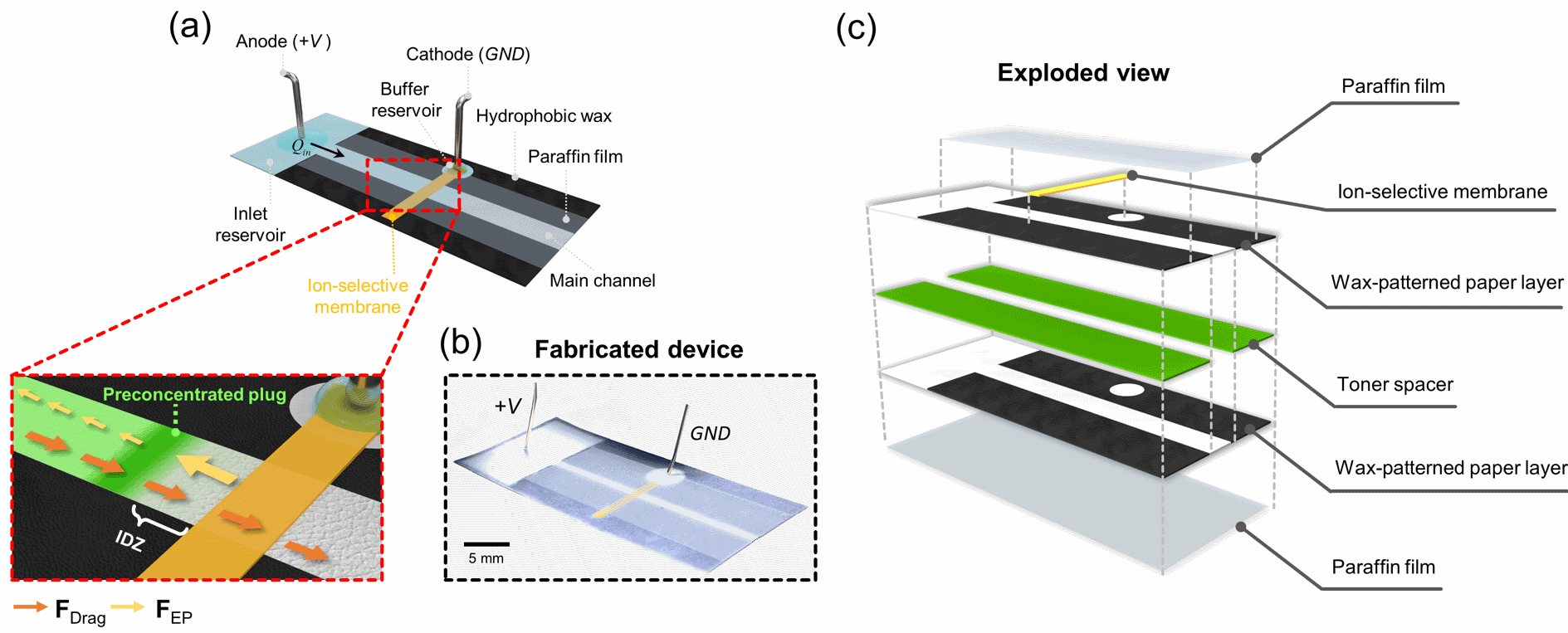
Figure 1. (a) Schematic diagram of paper-fluidic channel as a nanoelectrokinetic preconcentrator. Inset image shows the nanoelectrokinetically preconcentrated plug near the boundary of ion depletion zone, where the drag force (FDrag) and the amplified electrophoretic force (FEP) by the boosted electric field due to the lack of ions near nanoporous membrane are balanced. (b) Photograph of the fabricated nanoelectrokinetic uPAD. (c) Exploded schematic of the hierarchical capillarity-assisted nanoelectrokinetic preconcentrator which is fabricated by stacking multi-films.
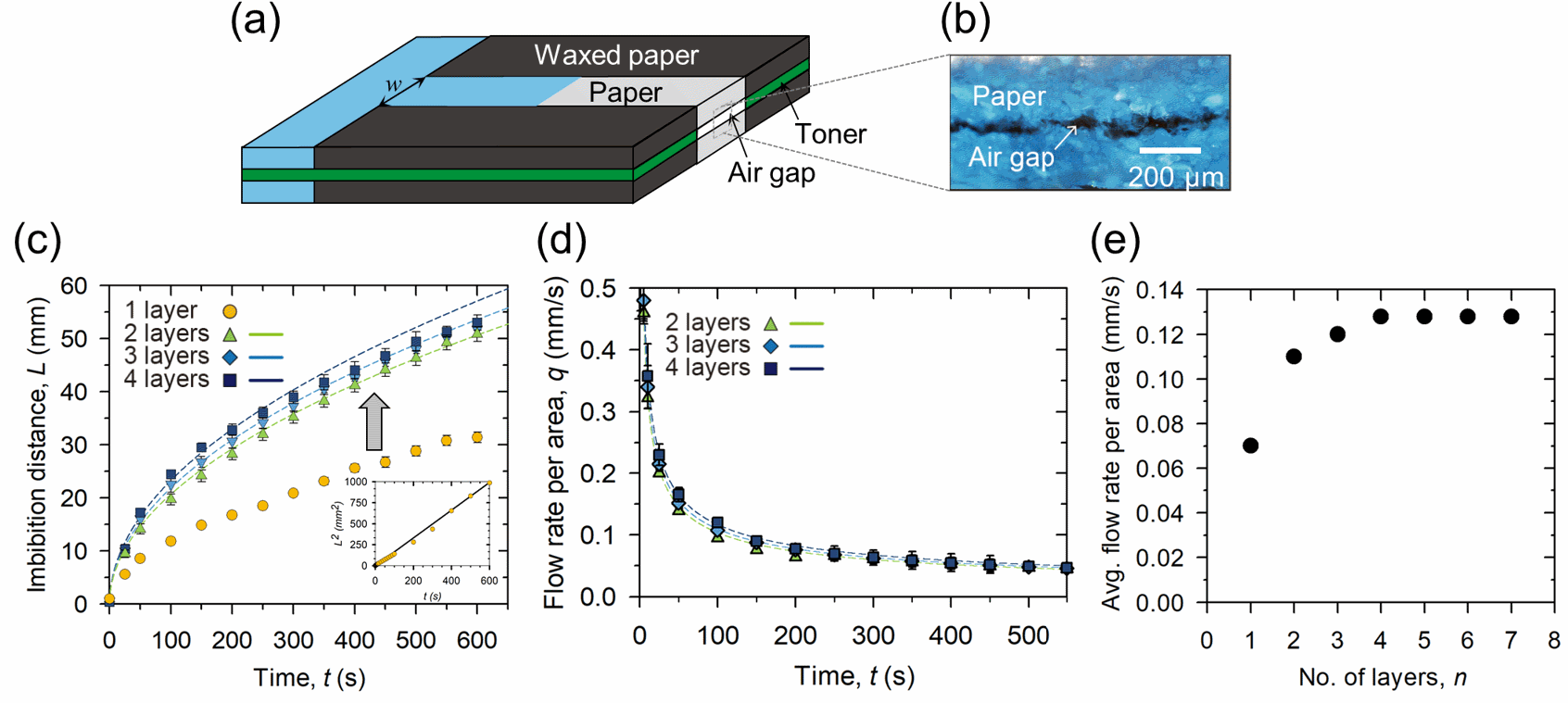
Figure 2. (a) Schematic illustrations of a multi-layer paper channel consisting of several paper layers aligned and adhered together in non-channel parts that are waxed papers with a toner. (b) Microscopy image of small air gaps created between the paper layer, measuring 10 um in height h. (c) Liquid imbibition distance, L, versus time, t, in multi-layer paper channels with a different number of layers n, such as n=1 (yellow circle), n=2 (green triangle), n=3 (blue diamond), and n=4 (navy square). The dotted colored lines show the theoretical imbibition distance in each multi-layer paper channel calculated according to equation (1). Inset: Square of the liquid imbibition distance versus time in a single-layer paper channel. The solid black line is the best-fitting line with a slope corresponding to DP2 and a value of 2.7 X 10-12 m2/s2. (d) Liquid flow rate per unit area, Q, versus time in multi-layer paper channels with different layers, from double to quadruple. The dotted colored lines represent the theoretical flow rate per unit area in each multi-layer paper channel. (e) The theoretical average flow rate per unit area between 1 to 600 s in n-layer paper channels in 1 ≤ n ≤ 7.
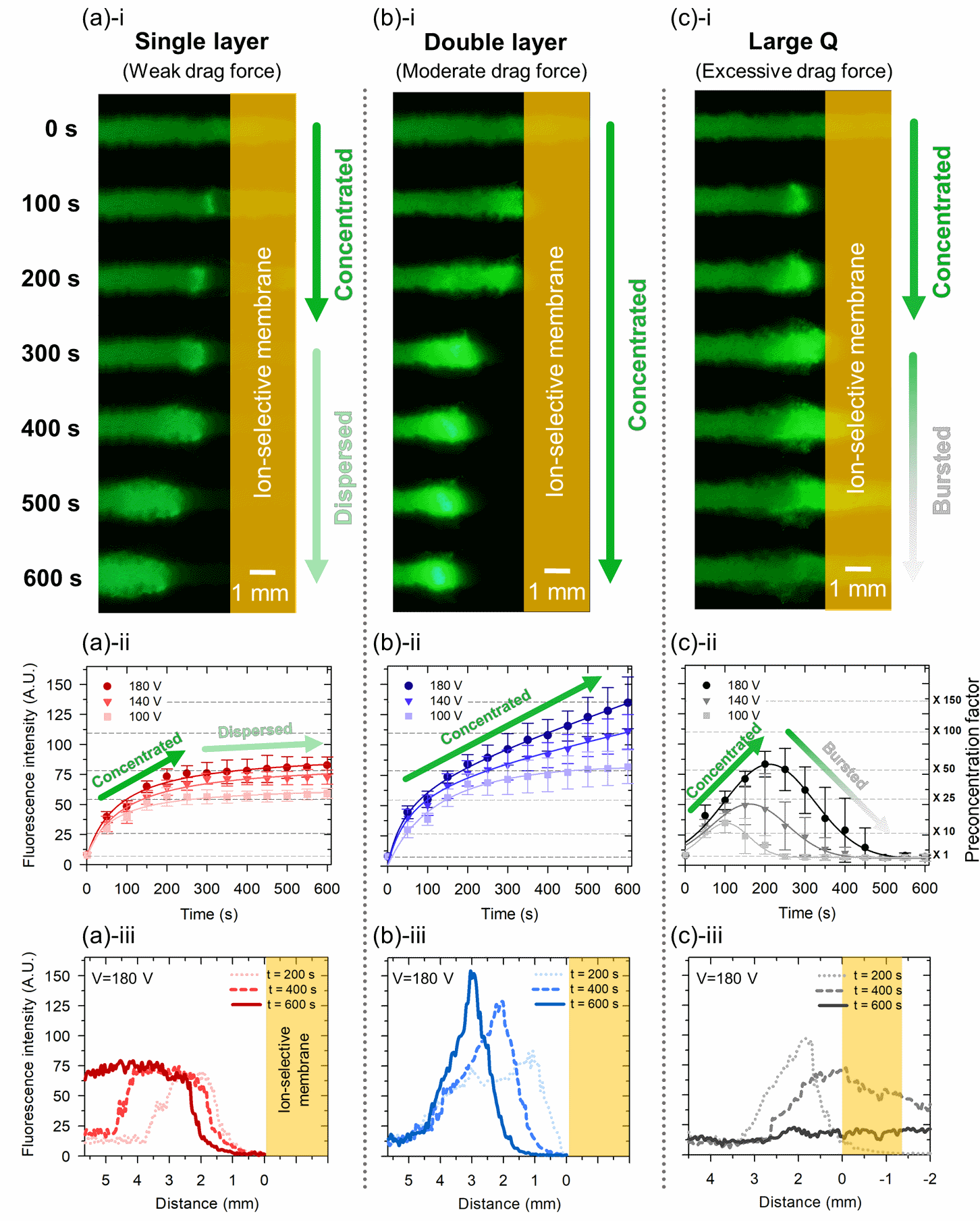
Figure 3. Experimental results of nanoelectrokinetic preconcentration depending on drag forces in (a) a single-layer device, (b) a double-layer device, and (c) a single-layer device with larger pores for significantly higher flow rate. For each column of (a)~(c), the first row (i) shows time series snapshot of preconcentration process for 10 min. In the second row (ii), averaged fluorescence intensity over time are measured from captured snapshots. 100 V, 140 V, 180V are chosen for operational voltage. The third row (iii) presents the fluorescence intensity plots as line profiles starting from the surface of the ion-selective membrane during the preconcentration process.
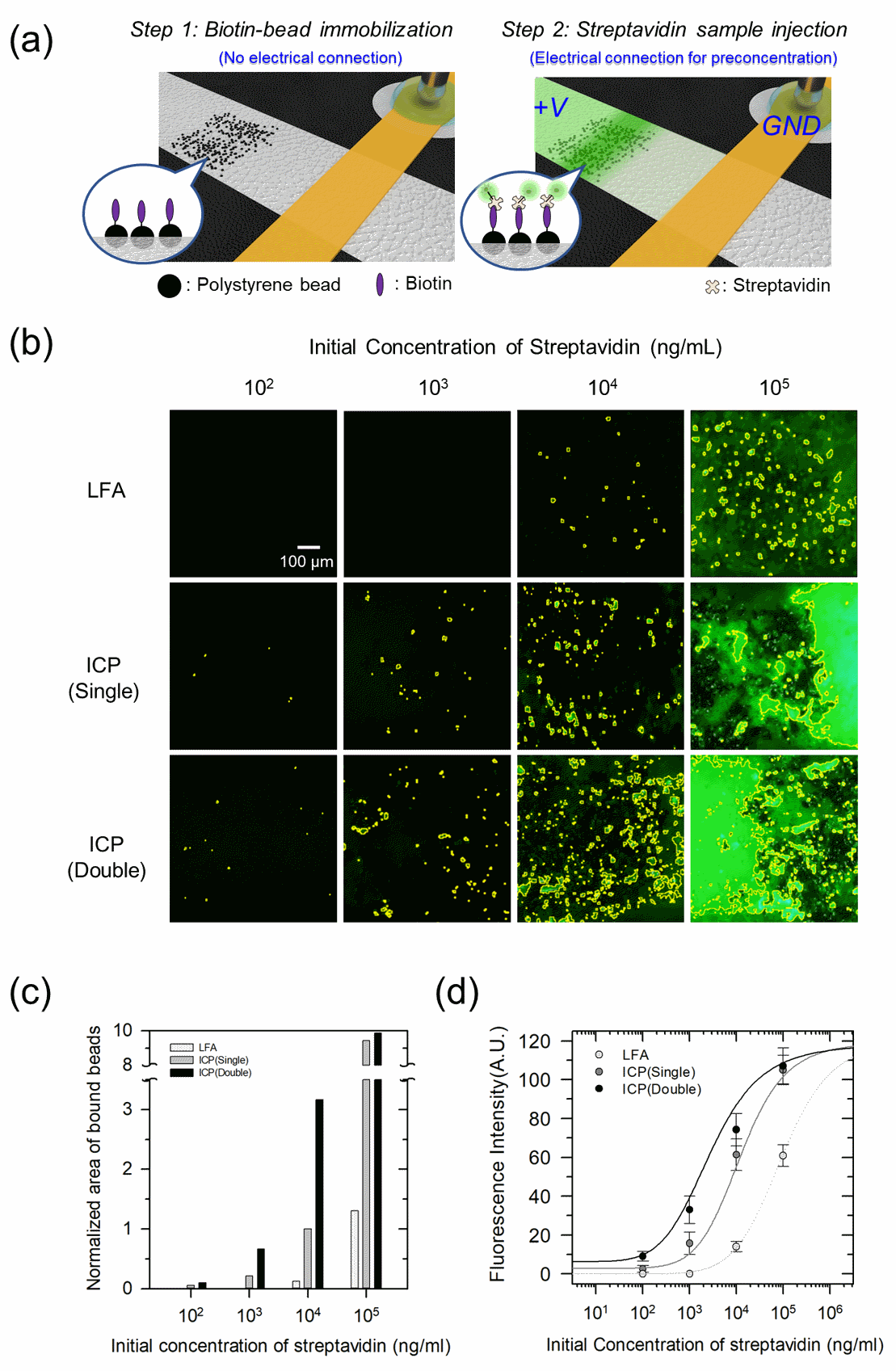
Figure 4. Demonstration of bead-based immunofluorescence assay with the hierarchical capillarity-assisted nanoelectrokinetic preconcentration. (a) Schematic diagram of immobilizing biotinylated beads (step1) and nanoelectrokinetic preconcentration of streptavidin labeled by fluorescent dye (step2). (b) Fluorescence microscopic images of bead-imbedded site in the lateral flow assay (LFA), single- and double-layer devices after preconcentration process (ICP). The yellow bounded regions represent binding signal from biotin-streptavidin complex. (c) The normalized area of beads brightened by the biotin-streptavidin complexation. After preconcentration, the normalized area of brightened beads expands by 300% in double-layer ICP device compared to single-layer ICP device except for excess streptavidin concentration of 102 ng/ml. (d) Binding curves are fitted by immunoassay data at the initial streptavidin concentration of 102 ng/ml, 103 ng/ml, 104 ng/ml and 105 ng/ml.